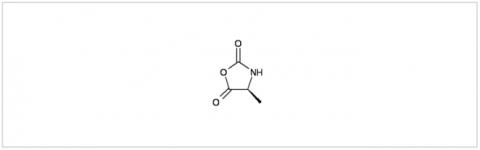
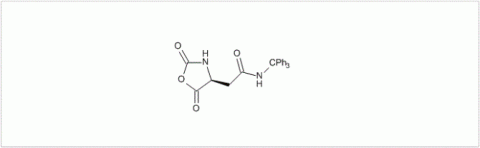
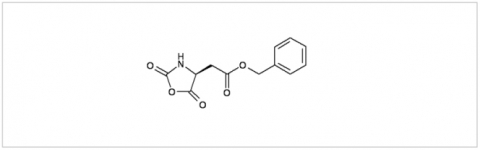
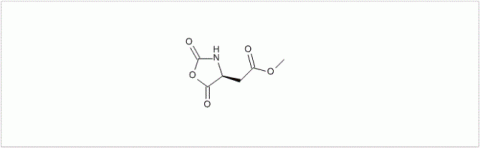
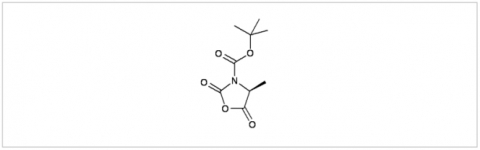
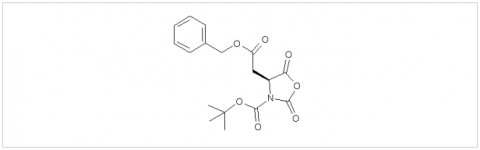
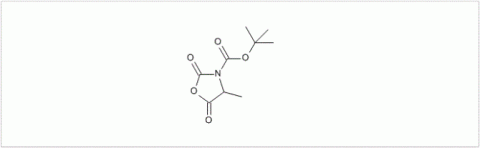
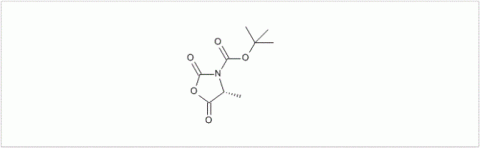
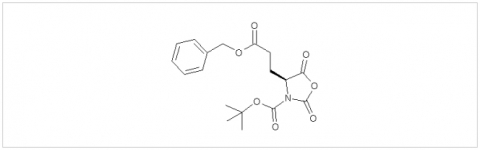
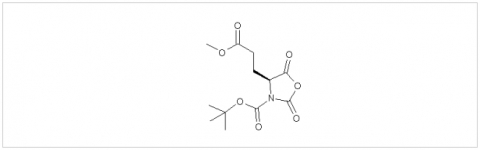
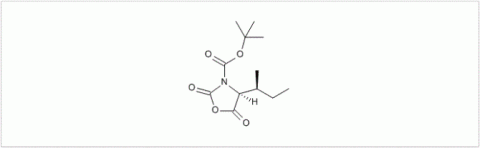
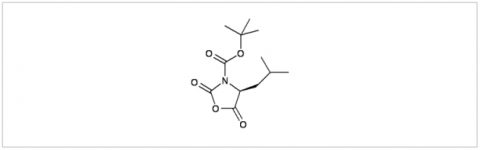
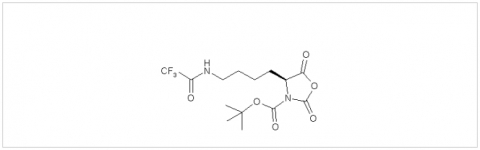
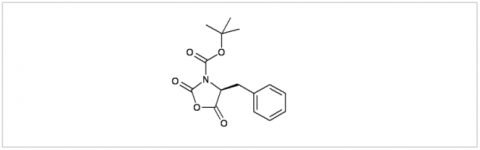
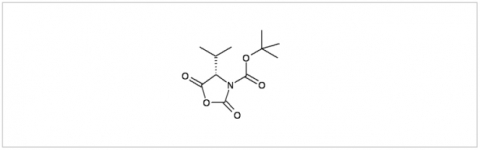
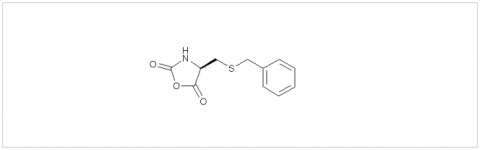
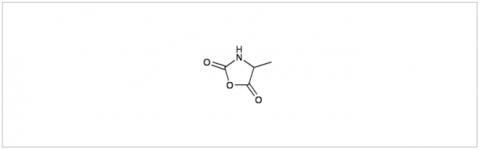
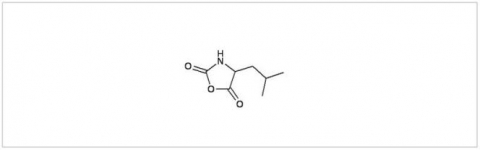
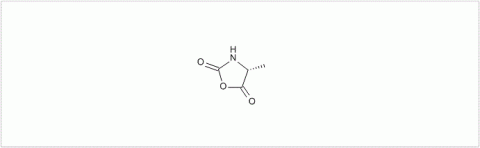
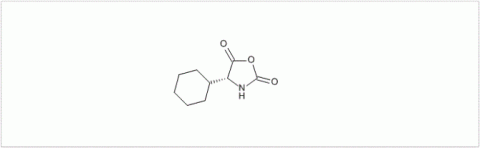
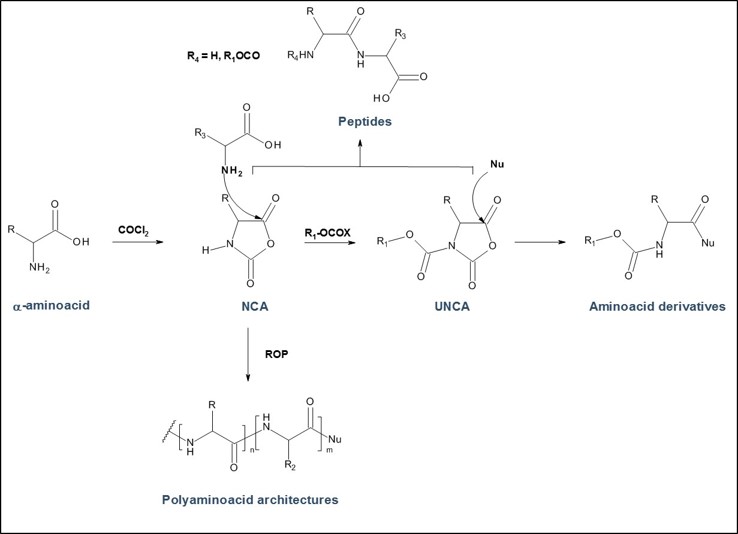
PMC Isochem produces at scales from gram to ton quantities of α-aminoacid N-CarboxyAnhydrides (NCAs) and Urethane N-Carboxyanhydride (UNCAs).
- monomers for Ring Opening Polymerization (ROP) for Poly-L-Lysine, Polysarcosine, Poly-L-Leucine, Poly-L-Glutamic acid and many block or statistic copolymers like Glatiramer Acetate
- powerful building blocks for smooth and eco-friendly coupling reaction with various nucleophiles for NCAs and UNCAs
PMC Isochem offers about 60 NCAs and the synthesis on demand of new NCAs or UNCAs including derivatives of non natural α-aminoacids.
Applications
Peptide based therapeutic polymers: NCAs are strategic building blocks for the preparation of polyaminoacid architectures for polymer drugs or polypeptide carriers in drug delivery and targeting technologies. Expanding the range of available NCA monomers including side chain functionalized α-aminoacids, offers post-polymerization modifications which broaden the perspectives of α-aminoacid based polymer therapeutics based on Polymer based on various polyaminoacids such as: Poly-L-Lysine, Poly-L-Glutamic acid, Polysarcosine...
α-aminoacids coupling in APIs synthesis: UNCAs are now an established and attractive alternative to traditional α-aminoacid activation strategies for coupling reactions. The strength of this technology for industrial processes is demonstrated by its lower environmental and safety impact when compared to other coupling agent strategies. It also offers product purity profile advantage due to the reaction selectivity and the absence of any coupling reagent.
Logistic solution and support
- Packaging: moisture proof double sealed bag.
- Transportation: frigipack for small quantities, insulated box with dry ice, reefer container for large quantities with temperature monitoring set.


- Stability and storage: storage under nitrogen, at temperatures of -20°C +/- 5°C or 0° +/- 5°C depending on the stability of the NCA.
- Handling: allow the package to warm up to room temperature before opening and handle under dry
For more detailed technical support, please contact us
Reference : Yves Robin, Chemistry Today, 33(4) July-August 2015