Custom manufacturing of Intermediates, APIs, Excipients and Polymers for drug delivery solutions
PMC Isochem offers cGMP and non cGMP industrial exclusive custom synthesis and provides tailor made services from development to production, including route selection, process and analytical development as well as process optimization.
Flexible Solutions: PMC Isochem's highly skilled teams interact closely with the pilot units and multipurpose facilities.
Reduced Time to Market: PMC Isochem's knowledge and management tools ensure an optimized timing for projects.
Continuous Cost Savings: PMC Isochem delivers substantial improvements in manufacturing and creates additional value by reducing operating costs.
Security of Supply Chain: PMC Isochem optimizes and secures sourcing of raw materials, based on total transparency with customers.
Custom manufacturing of APIs, GMP Excipients, Polymers for drug formulation like polyaminoacid (Poly-Sarcosine, Poly-Lysine, Poly-Glutamic acid or esters, Poly-Leucine, and many copolymers), Polymer drug conjugates
- cGMP (FDA) and non cGMP
- Clinical batch supply
- Routine production
- Supply chain
- Regulatory support
- Risk management
- Full transparency
Case studies
Preclinical and tox studies of an NCE
- Minimum R&D for secured scale-up from medicinal chemistry
- Solving a major stability issue of an intermediate
- Rapid roduction of 3 kg batch
Phase I advanced intermediate: 100 kg delivery
Achievement: development and scale-up from lab procedure
- Rapid development (avoiding 2 filtrations & use of DCM)
- Minimum R&D for secured scale-up
- Production of 100 kg batch 4 months after kick-off
- Then development and scale-up of a second generation process
Pre-launch regulated intermediate: multiton production
Achievement: Rapid industrial technical transfer
- Demonstration run completed 3 months after the kick-off
- Full size batch production 4 months after the kick-off
- Product quality – Supply chain & QA support
- Yield and production improvement over the following 12 months
Generic API: new supplier
Achievement: development and filing of API
- Process developed from scratch (2 steps)
- Analytical development and validation in addition to Pharmacopoeia
(GTI’s and residual solvents)
- Scale-up and process validation achieved within 9 months
- Full filing data delivered to the customer in time
Tolling hydrogenation of an intermediate
Achievement: rapid tech transfer and production
- Familiarization and scale-up preparation within 1 month
- 2 successful batches the next month
- 10 MT campaigns routinely
Devlopment and scale up of an catalogue molecule
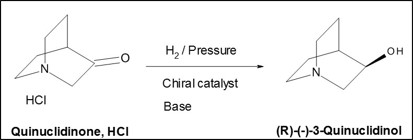
(R)-(-)-3-Quinuclidinol is a leading example of our catalogue offer, the result of successful development and scale-up of a performing chiral process using a catalyst developed in a partnership.
For more detailed technical support, please contact us